Engine cooling is an important part of keeping the motor running at the perfect temperature and preventing overheating. When your engine is running, friction is made from all the moving parts which create heat. Excess heat in the engine will cause damage such as blown head gaskets, damaged seals, damaged piston rings, and in the worst-case scenario crack the engine block. The cooling system prevents the engine from overheating by removing excess heat from the engine.
There are three different cooling systems that are used on gasoline engines.
- Air Cooled
- Oil Cooled
- Water Cooled
- Air Cooled systems work by expanding the surface area or increasing the flow of air over the object to be cooled, or both. An example of the former is to add cooling fins to the surface of the object, either by making them integral or by attaching them tightly to the object’s surface (to ensure efficient heat transfer). In the case of the latter, it is done by using a fan blowing air into or onto the object one wants to cool. The addition of fins to a heat sink increases its total surface area, resulting in greater cooling effectiveness. This type of cooling can be found mostly on small engines used in lawnmowers and lawn and garden tractors and some motorcycles. Motorcycles however use a combination of air and oil cooling.
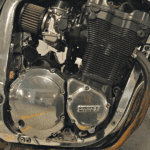
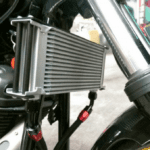
- Oil Cooled system uses engine oil as a coolant, typically to remove surplus heat from an internal combustion engine. The hot engine transfers heat to the oil which then usually passes through a heat exchanger, typically a type of radiator known as an oil cooler. The cooled oil flows back into the hot object to cool it continuously. This type of cooling is found mostly in motorcycles where space is limited to run a water cooling system. Motorcycles will use a combination of air and oil-cooled systems to reduce engine heat.
- Oil Cooled Engine
- Oil Cooler
- Water Cooled systems use a method of heat removal from components and industrial equipment by using fluid to run through the passages of the engine block and cylinder heads to cool and remove heat. In the case of an automobile engine, the fluid is run through the radiator and then into the passages of the engine. In the engine, the fluid will absorb heat and then returns to the radiator where the heat is removed before traveling through the engine again. This constant travel of fluid is what keeps the engine cool and prevents overheating.
All of today’s automobiles will use water-cooled systems. Of course, there are more parts involved to keep this system running properly and efficiently. Also using the correct fluid in your automobile’s cooling system is a vital part of the engine cooling process. The use of Antifreeze as the liquid coolant is extremely important to keeping your car running cool and efficiently without the risk of overheating the fluid or the risk of the coolant liquid freezing in cold temperatures. Hence the name Antifreeze.
The Coolant Paradigm
In today’s modern internal combustion engines using a water-cooled system, having the proper engine coolant is an extremely important part of your engine’s cooling system. The engine cooling system uses liquid to cool the engine. Common sense would tell us that a water-cooled system must use water as the cooling fluid. This is somewhat correct which will be explained further down in this article.
| Note: Using the 2004-2006 Pontiac GTO as an example, which uses a Chevrolet LS, water-cooled engine, the optimal engine temperature for power and efficiency is around 210º F. |
Using just water would seem like the logical choice to add to a water-cooled system. Water itself as an engine coolant could lead to some serious problems as will be discussed. Water will boil at 212º F (100º C) at normal atmospheric pressure. Using just water as engine coolant means you are in danger of actually boiling the engine coolant should your engine’s temperature rise above 212º F. While sitting in traffic on a hot day your engine can easily reach temperatures of 220-230º F which would cause the water coolant to boil. When any liquid boils, the fluid will turn into vapor. The vapor will actually push the coolant away from the metal it is trying to cool. Coolant vapor has zero cooling properties, so your engine will absolutely begin to overheat. As the temperature of the engine rises, the metal parts of your engine will become damaged including pistons, valves, water pump, and can include warping the cylinder heads and cracking the engine block.
Using just water as an engine coolant in cold climates will also lead to some serious engine problems and mechanical failures. Water freezes at 32º F (0º C). If you live in a cold climate where temperatures reach below 32º F (0º C), your water engine coolant will turn into ice, and when water freezes it expands. This means once your water coolant has frozen to ice, your radiator will crack, the coolant reservoir will crack, the water pump will crack, radiator hoses will crack, and anywhere else in the engine water coolant is being stored. This includes the engine block. Frozen water in an engine can easily crack an engine block. You may think your engine is made of strong iron or aluminum, but ice will prove it is stronger especially when trapped in an area.
Lastly, straight water in the cooling system can cause rust and corrosion. Metal and water never have and never will play well together. Water on metal causes rust. With water comes dirty water. Water doesn’t stay clean forever. The water will become dirty over time causing metal parts to form rust on them. The radiator, the engine, and cylinder heads, all contain metal parts that can become rusted over time, especially if the car has been sitting for a while not being run or driven. Corrosion and rust are something no car enthusiast ever wants to see.
The Importance of Antifreeze
We know liquid or fluid is used in a water-cooled system, but what fluid should be used? This is where Antifreeze or otherwise known as engine coolant comes in. Antifreeze unlike water has very different chemical properties. Antifreeze engine coolant actually raises the boiling point of the fluid and lowers the freezing point of the fluid. The irony is, Antifreeze actually mixes with water to accomplish this. Water has amazing qualities for cooling and is still nature’s greatest gift. Water has a high boiling point, low freezing point, and collects heat fast, and removes heat fast. Unfortunately, internal combustion engines also create a great deal of heat and need to be cooled slightly faster than what water can offer, and nature can give us extremely cold weather that can freeze water very quickly.
Antifreeze engine coolant solves the problem of both boiling and freezing points to accommodate the extreme heat of the internal combustion engine while battling the rigors of severe freezing weather. Antifreeze is made of distilled water along with a chemical called ethylene-glycol. Antifreeze can be purchased as a concentrate meaning it must be mixed with water, or as a ready-to-use fluid that is already mixed as a 50/50 (50% antifreeze, 50% distilled water) fluid. The use of Antifreeze coolant drastically improves the boiling and freezing points of the water as seen by the image below:

As stated earlier, just plain water by itself boils at 212º F (100º C) and freezes at 32º F (0º C). The use of a 50/50 mix of Antifreeze raises the boiling point to 265º F (129º C) and lowers the freezing point to negative -34º F (-37º C). The big difference is in the freezing protection which keeps the fluid from freezing inside your radiator and engine which will cause damage. It not only ensures your engine stays within a safe operating temperature range, but it also keeps the coolant from freezing.
Lastly, Antifreeze helps to keep the engine clean and rust and corrosion-free. The chemical makeup of antifreeze helps to keep the radiator, water pump, and engine corrosion-free and prevents the build-up of “gunk” that can form over time. This of course makes all the cooling system parts last much longer and optimally prevents pre-mature repair.
Orange Antifreeze vs. Green Antifreeze
The 2004-2006 Pontiac GTO uses orange Dex-Cool Antifreeze. Some of you may be asking yourself why can’t I use the green antifreeze, it’s the same stuff? Well, actually they are not the same. I’m going to sum this up as easily as possible.
- Green Antifreeze: Green engine coolants are designed for use in older cars (pre-2000), ones that contain a lot of steel and copper components in the cooling system. To protect these metallic parts from rust and corrosion, Inorganic Additive Technology (IAT) gets added to the mix.
- Orange Antifreeze: Orange antifreeze, which also defends against corrosion is made for newer cars with more aluminum and nylon parts in the cooling system. The transition from steel and copper to aluminum and nylon in engines started back in the 1990s. Due to this change, GM introduced DexCool. DexCool is a type of coolant that uses a mix of different Organic Acid Technologies (OAT) to help to help inhibit the buildup of rust and corrosion.
The 2004-2006 Pontiac GTO comes with an aluminum engine block with aluminum cylinder heads. This is why it is so important to use the correct coolant for your vehicle. DexCool antifreeze is specially designed to be used on aluminum engine parts. I recommend just using the “ready to use” 50/50 mix. It costs a little bit more than if you purchase the concentrate version, but it is already mixed from the factory using distilled water. Also using the ready-to-use version ensures you have the correct mixture in your vehicle for optimal cooling and antifreeze performance.
| Note: Antifreeze is extremely poisonous to animals Ethylene-Glycol is the ingredient that can be fatal to any animal. Ethylene glycol is so dangerous that just 2 teaspoons of the liquid are lethal for a cat and a couple of tablespoons can kill a medium-sized dog. Please take extreme caution when pouring and disposing of Antifreeze. Clean up any spills immediately and dispose of the clean-up rags or towels used safely and out of reach of animals and pets. |
Components Of The Cooling System
The cooling system’s main function is to regulate the operating temperature of the engine and prevent it from overheating. The cooling system is operated by using many different parts to properly maintain the operating temperature of the engine while it is running. If any of these parts were to fail or break, it would result in the overheating of the engine which can lead to mechanical or catastrophic failure of the engine. The following parts are used in a gasoline engine water-cooled system:
| PART | DESCRIPTION |
| Radiator: | The radiator stores the engine coolant as well as removing the heat from the coolant fluid by utilizing a combination of air and cooling fans. Air is passed through the fins of the radiator reaching the radiator core which passes the engine coolant through the cooling area before making its return to run back through the engine to repeat this procedure. |
Radiator Hoses: |
The radiator hoses attach to the radiator and carry the engine coolant through the water pump and make its return through the thermostat housing. One hose is for the coolant exit from the radiator, the other hose would be the coolant return back to the radiator from the engine. |
| Coolant Reservoir: | The coolant reservoir stores extra engine coolant to feed the radiator should the coolant level of the radiator become low. It also works as an overflow should the coolant level of the radiator become too full. You can add new coolant to the system by filling the coolant reservoir should the level of the reservoir become low. |
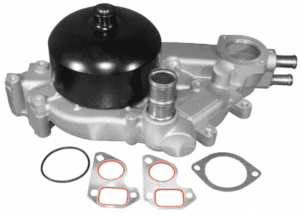
| Water Pump: | The water pump does exactly what it says, pumps water (coolant). The water pump propels or “pumps” the coolant used in the engine back into the radiator to be cooled, then through the lower radiator hose and through the thermostat to return through the engine. This is a continuous cycle to cool the engine. On the 2004-2006 Pontiac GTO, the water pump also houses the thermostat. |
| Thermostat: | The thermostat sits in the thermostat housing which connects to the lower radiator hose coming from the radiator. It acts as a valve to prevent engine coolant from running through the engine until it reaches a certain temperature. Once the thermostat reaches the given temperature, the valve on the thermostat will open, allowing the coolant to pass through the thermostat and make its return to cool the engine. By letting the engine warm-up as quickly as possible, the thermostat reduces engine wear, deposits, and emissions. |
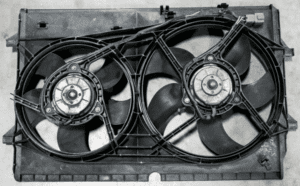
| Cooling Fans: | The cooling fans sit in front of the radiator and push air through the radiator cooling fins and to the radiator core to help cool the coolant release heat before it enters the engine. The cooling fans are set to come on when a certain temperature is reached. The fans will not always be running. The cooling fans act as a backup to further cool the engine coolant when above normal engine temperatures are reached. The cooling fans will come on for example if sitting in stop-and-go traffic on an extremely hot day, and also when using the air conditioning because the engine works a little harder to run the air conditioning system. |
| Antifreeze/Coolant: | Antifreeze engine coolant is used as the fluid to cool the engine. Antifreeze has much lower freezing points to prevent the coolant from turning to ice or freeze in cold temperatures and also has higher boiling points to prevent the engine coolant from overheating and not cooling the engine as it should. Antifreeze also keeps engine parts from rusting and forming corrosion, which prolongs the life of the engine and the cooling system parts that are used by it. |
How The Cooling System Works
We already know using engine coolant is what helps to keep the engine running at the optimal temperature. Running an engine too cool can result in a loss of power and efficiency. Gas, Oil, and even some of the parts in the engine require heat to work properly. Running an engine too cold can result in the engine becoming gummed up and gunk and deposits forming in the engine because the engine does not get hot enough to burn and expel these deposits. Running an engine too hot can result in engine failure from the engine parts becoming overheated and damaged such as seals and gaskets. Also, aluminum engine parts such as the engine block and cylinder heads can warp and even crack if the temperature exceeds what the parts can handle.
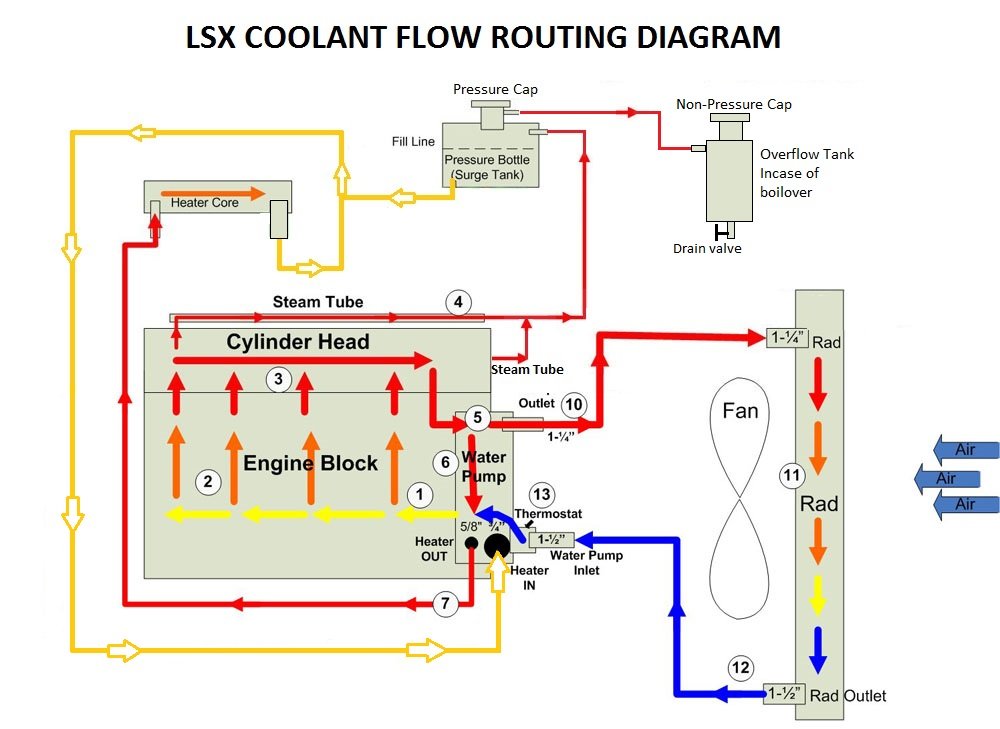
The diagram below will show you exactly how the cooling system works and the importance of its function for a water-cooled engine:
- The radiator holds and stores the engine coolant
- Blue represents the path of the cool coolant entering the water pump through the thermostat housing. This coolant will enter through the lower radiator hose.
- Yellow represents the coolant get warming as it runs through the engine block
- Orange represents the coolant picking up more heat and getting hotter
- Red represents the hot coolant running through the engine before it is passed through the water pump and back to the radiator.
- Once the coolant reaches the radiator, the hot coolant is cooled down and expels the heat it has picked up, and then the process is repeated.
- Coolant is also run through the heater core which is how your vehicle brings heat to the cabin area inside your vehicle.
| Note: I hope you found the information contained on this website useful. Many hours of time and research have gone into building this website. Please feel free to donate to Mark Quitter Racing. Donations will be used to pay for website services or for any other practical use per your request which can be filled out on the PayPal donation link. There is no minimum amount, and your contribution is greatly appreciated. Donate here. |
Sign up for Speed Perks Rewards to save on Gas at Advance Auto Parts today!